Earth is 71% water
97% of which is saltwater
3% of this water is freshwater
2.5% of freshwater is locked in lakes, and groundwater
0.5% of freshwater is used for consumption around the world!
The Geometry of Polarity
Water is not a linear molecule. Due to the two pairs of unshared electrons on the oxygen atom, the hydrogen atoms are pushed together, resulting in a bent molecular geometry
The bond angle between the hydrogen atoms is approximately 104.5°. This specific shape is what makes the molecule polar; if water were linear, the partial charges would cancel each other out. This asymmetry is the foundation for almost every biological process, from protein folding to the formation of cellular membranes
Grade and Purity in the Lab
Not all H2O is created equal. In pharmaceutical research, the "Grade" of water used can make or break an experiment:
Type III Reverse Osmosis Glassware rinsing, water baths
Type II Deionization Buffer preparation, general chemistry
Type I (Ultrapure) Ion Exchange/UV PCR, HPLC, cell culture, and mass spec
The Molecular Science of Water (H2O)
Water is often called the "universal solvent," but from a biochemical perspective, it is a structural masterpiece. Its unique behavior stems from its simple atomic composition and the complex electrical charge distribution that follows.
Atomic Structure and Covalent Bonding
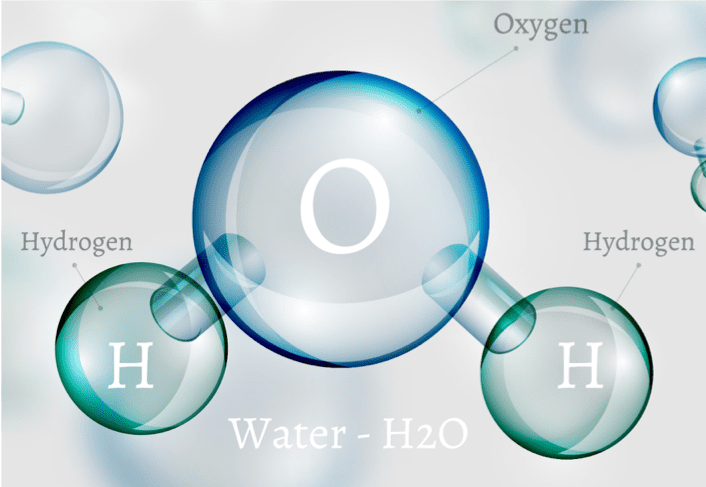
A single water molecule is formed by one oxygen atom and two hydrogen atoms. These atoms are held together by polar covalent bonds.
In this arrangement, the oxygen atom is highly electronegative, meaning it has a much stronger "pull" on electrons than the hydrogen atoms. As a result, the shared electrons spend more time orbiting the oxygen nucleus, creating a dipole moment.
Oxygen atom: Becomes partially negative
Hydrogen atoms: Become partially positive
Biological Significance
In the context of life sciences, water is the medium for all intracellular reactions. Its polarity allows it to dissolve salts, sugars, and acids. Understanding water’s physical chemistry is essential for precision research
Applications in Biotechnology and Lab Research
In drug development, water’s polarity drives the hydrophobic effect. When non-polar molecules are placed in water, the water molecules organize into a highly ordered "cage" around them to maximize hydrogen bonding
Protein Folding: This is the primary force behind protein folding; hydrophobic amino acid side chains are pushed into the interior of the protein to minimize contact with water, while hydrophilic chains face outward
Drug Binding: Many therapeutic lead compounds are designed to "displace" these ordered water molecules within a target protein's binding pocket, a process that provides a significant thermodynamic boost to binding affinity
Hydrogen Bonding: The "Molecular Glue"
Because of its polarity, water molecules are attracted to one another. The partially positive hydrogen of one molecule is attracted to the partially negative oxygen of a neighbor. This attraction is known as a Hydrogen Bond
While an individual hydrogen bond is weak compared to a covalent bond, the collective strength of billions of these bonds gives water its remarkable properties
High Specific Heat: It takes significant energy to break these bonds, allowing water to stabilize temperatures in both the environment and the body
Cohesion and Adhesion: Water "sticks" to itself (cohesion) and other surfaces (adhesion), enabling capillary action in plants
Density Anomaly: Unlike most substances, water becomes less dense when it freezes. The hydrogen bonds lock into a crystalline lattice that pushes the molecules further apart, which is why ice floats